| Open Access | Peer Reviewed | Original Research |
Pre-Harvest Oxalic Acid Application Improves Fruit Size at Harvest, Physico-Chemical and Sensory Attributes of ‘Red Flesh’ Apricot During Fruit Ripening

Copyright: © 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License. J. Hortic. Sci. Technol. © 2021 Pakistan Society for Horticultural Science.
ABSTRACT
Apricot is a highly nutritive stone fruit which ripens quickly after harvest and exhibits rapid fruit quality deterioration at ambient conditions. The current research work was executed to evaluate the effect of pre-harvest application of oxalic acid (OA); (0, 0.5, 1, and 2mM) on fruit quality of ‘Red Flesh’ apricot during fruit ripening at shelf under ambient conditions (25±1 °C; 60-65% RH). Fruit size, average fruit weight, fruit juice percentage was determined at harvest. While, fruit weight loss, fruit colour, fruit firmness, titratable acidity (TA), soluble solid contents (SSC), ratio of SSC to TA, ascorbic acid, total phenolics content (TPC), anti-oxidative scavenging activity (ASA), superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) enzymes activity were determined during fruit ripening. Fruit sensory attributes (fruit pulp colour, fruit taste, fruit flavour and overall acceptability were determined at fruit ripening. Pre-harvest application of 2mM-OA was found the most effective in improving fruit size, average fruit weight, juice percentage at harvest, about 5%, 6% and 15% higher than control, respectively. Additionally, pre-harvest application of 2mM-OA retained higher fruit firmness, ascorbic acid, TPC, ASA and activities of POD, CAT enzymes in apricot fruit during fruit ripening at ambient conditions. On day-5 of fruit ripening 2mM-OA-treated apricot fruit exhibited about 28%, 20%, 17%, 7%, 9% and 23% higher fruit firmness, ascorbic acid, TPC, ASA and activities of POD, CAT enzymes, respectively as compared to control. Moreover, significant lower fruit weight loss (35%), SSC (20%) and SSC:TA ratio (30%) were exhibited by the apricot fruit treated with 2mM-OA than unsprayed apricot fruit. However, fruit treated with 1 mM-OA exhibited better fruit sensory attributes compared to other treatments at fruit ripening. Conclusively, pre-harvest application of 2 mM-OA improved the fruit size, delayed fruit ripening, and retained higher fruit antioxidants of apricot at ambient conditions.
INTRODUCTION
Apricot, a stone fruit with soft and fleshy mesocarp belonging to Rosacea family is being grown in more than 40 countries throughout the temperate regions of the world (Chadha, 2003; Aubert and Chanforan, 2007). Apricots are generally cultivated in the Northern Hemisphere, the Mediterranean and Central Asia regions contributing more than 80% of the world’s apricot production (FAOSTAT, 2019). Turkey, Uzbekistan, Algeria, Iran, and Pakistan are the top world leading apricot producing countries. Pakistan ranks at 5th spot in world’s apricot fruit production, contributing about 2.5% of world’s apricot. During 2019, the country produced about 105 thousand tonnes of apricot fruit on an area of about 20 thousand ha (FAOSTAT, 2019). Within the country, Balochistan province shares most of the apricot fruit production, about 93% of the country’s total production (MNFSR, 2017). Balochistan province is also the home of the very diverse apricot genotypes. According to a report about 60 types of the apricot commercial cultivars are being grown in the province (Ali et al., 2011). Moreover, apricot fruit is a rich source of phenolic, phytochemicals, antioxidants, and carotenoids, which are important for human health (Ruiz et al., 2003).Despite huge production volume of apricot fruit, the country does not rank in top apricot exporters due to massive postharvest losses. Moreover, apricot has a limited postharvest life at ambient conditions experiencing rapid fruit ripening and deterioration after harvest (Yan et al., 2017). Although low temperature storage facility is used to delay the rapid postharvest deterioration (Agar et al., 2006). However, the core areas of apricot production lack this facility. Therefore, alternate approaches to reduce these losses at ambient conditions includes application of chemicals for delaying of fruit ripening.
The application of oxalic acid (OA) to delay fruit ripening has received much attention. Previously, OA has shown to have antioxidant activities, thus, it plays an important role in systemic resistance, programmed cell death, redox homeostasis in plants, and an anti-senescence effect in many fruits (Ding et al., 2007; Zheng et al., 2007; Wu et al., 2011). Application of OA have been reported to suppress quality deterioration, delay fruit senescence and in reduction of postharvest disease development in peach, mango, litchi, and jujube fruit (Zheng and Tian, 2006; Zheng et al., 2007; Wang et al., 2009; Zheng et al., 2011). Moreover, OA has also been reported as an anti-browning agent in fresh cut apple and banana slices (Son et al., 2001; Yoruk et al., 2002). To the best of our knowledge, very limited work has been reported in literature, exploring oxalic acid application on postharvest fruit ripening and fruit quality at ambient conditions. This report would be the first time to study the effect of pre-harvest application of OA on apricot fruit quality during postharvest fruit ripening. So, the current study was carried out to investigate the impact of pre-harvest OA application on apricot cv. Red Flesh quality at ambient conditions.
MATERIALS AND METHODS
Plant material and experimental design
The experiment was conducted in a commercial apricot orchard located in Turkhezi, Balochistan, Pakistan (68°59′63″ E; 30°38′06″ N, located about 800 km in West-South from Islamabad) to check the effect of pre-harvest oxalic acid (OA) application on fruit size and physico-chemical characteristics of apricot during ripening. Apricot (Prunus armeniaca L. cv. ‘Red Flesh’) plants, an early season cultivar exhibiting good fruit quality fruits and appreciated by the consumers were used. The experiment was executed during 2018-19 in Spring-Summer period. Date of full bloom was 2nd March 2019, for the cultivar under study. Four trees of about eight years age grafted on local apricot seedling planted in square system at 6 m distance in East-West direction having uniform vegetative growth free from diseases and pest attack were selected for each treatment and subjected to foliar application of OA at fruit set stage during 2018. The experimental treatments included 0, 0.5, 1- and 2-mM OA. The trees sprayed with water only were taken as control. The experiment was executed under Randomized Complete Block Design (RCBD) arrangement with four replications. Single tree served as an experimental unit (four trees per treatment). For quantification of various physical and biochemical attributes the treated fruit were harvested at physiological maturity and immediately transported to Postharvest Science and Technology Lab, MNS-University of Agriculture, Multan, Pakistan. The physical parameters of fruit were determined at harvest, while for biochemical and anti-oxidative parameter quantification the fruit were retained at ambient conditions (25±1 °C; R.H. = 60-65%) up to day-5 of fruit ripening. Following parameters were recorded:
Determination of fruit physical characteristics
For measurement of fruit size and average fruit weight, 15 fruits were randomly selected per replication (60 fruit per treatment) and calculated with the help of digital vernier calliper (TESA Technology, Germany) and weighing balance (OHASU Corporation, USA), respectively. Average values of fruit size and weight were expressed in respective unit of mm and g. Fruit weight loss was calculated by using the method of Ullah et al. (2013) and expressed in percentage. Apricot fruit ground colour and fruit firmness were determined subjectively by using a scale ranging from 1 to 5 and expressed in numeric score. In case of fruit ground colour, the score 1 to 5 denoted by score 1 (100% green, 0% yellow colour) to score 5 (0% green, 100% yellow colour fruit), respectively. However, score 1 to 5 indicated by fruit firmness ranging from score 1 (100% firm, 0% soft fruit) to score 5 (0% firm, 100% soft) apricot fruits.
Determination of fruit biochemical characteristics
Soluble solid contents (SSC) of apricot fruit juice were determined by using digital refractometer (PAL-1, Atago, Tokyo, Japan) and were expressed in °Brix. Fruit titratable acidity (TA) and ascorbic acid were determined with titration using 0.1 N NaOH and dye (2,6-dichlorophenolindophenol) as reported by Ullah et al. (2013), respectively. TA and ascorbic acid were conveyed in respective units of % of malic acid and mg 100g-1. The ratio of SSC: TA was calculated by dividing SSC with respective TA and expressed in ratio.
Determination of anti-oxidative characteristics
Apricot fruit pulp antioxidants scavenging activity (ASA) and total phenolic contents (TPC) were extracted from stored samples of frozen pulp (-20 °C) and determined as per procedure given by Ullah et al. (2013). The ASA and TPC were expressed in respective unit of % inhibition of free radical 2,2 diphenyl-1-picrylhydrazil (DPPH) and mg GAE 100g-1. Briefly, 50 µL supernatant of apricot pulp tissue was mixed with 5 mL 0.004% methanolic solution of DPPH, incubated in dark condition for half hour followed by taking absorbance at 517 nm. TPC from apricot pulp tissue were determined by reacting the supernatant with Folin-Ciocalteu reagent and freshly prepared Na2CO3. After thorough mixing and incubation at room temperature the absorbance was taken at 765 nm. Similarly, apricot fruit pulp superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) enzymes activities were extracted from frozen pulp samples (-20 °C) as proposed and determined by the protocol of Ullah et al. (2013).
Determination of fruit sensory attributes
Organoleptic evaluation for flavour, texture, aroma, pulp colour and overall acceptability of the apricot fruit were executed at fruit ripening on day-5 of shelf. A panel of ten trained judges was employed to execute apricot fruit sensory evaluation by using Hedonic scale as described by Pilgrim and Peryam (1957).
Statistical analysis
The effect of pre-harvest OA treatment for calculated parameters were analysed through one-way (fruit physical at harvest and sensory attributes at fruit ripening) and two-way ANOVA (biochemical and anti-oxidative parameters at shelf) using computer-based software. Treatment means were tested with LSD test at P ≤ 0.05.
RESULTS
Fruit physical parameters
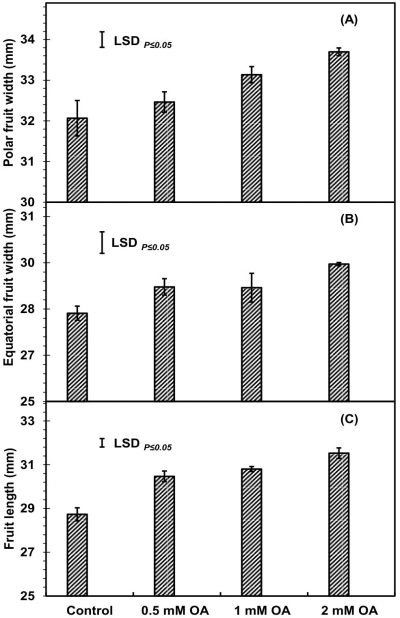
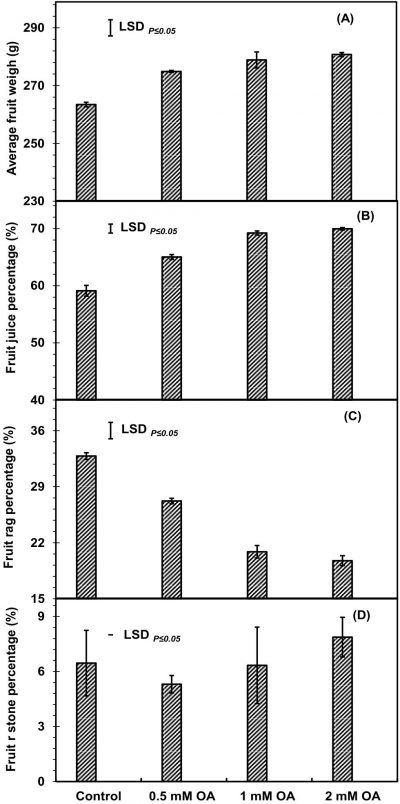
Pre-harvest OA treatment significantly increased apricot fruit size (fruit polar, equatorial width and length) as compared to control fruit at harvest (Fig. 1A-C). Apricot fruit size increased with increase in dose of OA. Fruit plants treated with 2mM-OA exhibited about 4.9-, 5.7- and 8.9-percent increase in polar, equatorial width and length of fruit as compared to untreated fruit, respectively. Significantly higher average fruit weight and juice percentage, while lower rag percentage were reported in 2 mM OA-treated fruit as compared to control fruit (Fig. 2A-C). However, fruit stone percentage was non-significantly affected by pre-harvest application of OA (Fig. 2D). The increase in average fruit weight, juice percentage and reduction in rag percentage were OA-concentration dependent. At harvest, about 6- and 15-percent increased respective apricot fruit weight and juice weight, while, about 38% less rag percentage were observed in 2mM OA-treated apricot fruit as compared to untreated fruit.
Figure 1: Apricot fruit size [polar width (A), equatorial width (B) and length (C)] as affected by pre-harvest application of oxalic acid. Vertical bars denote standard error (SE) of means. n= 30.
Figure 2: Apricot average fruit weight (A), juice percentage (B), rag weight (C) and stone weight (D) as affected by pre-harvest application of oxalic acid. Vertical bars denote SE of means. n= 30.
Fruit weight loss, colour, and firmness
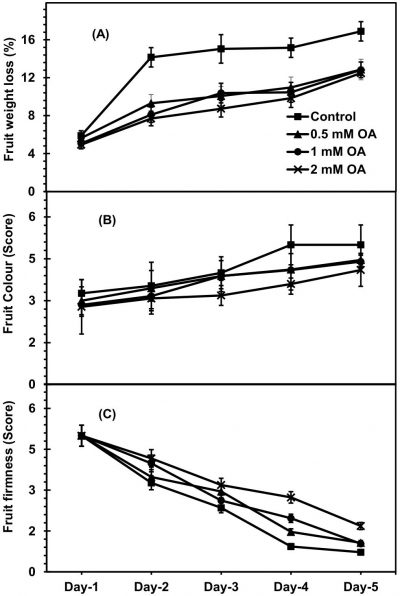
Both OA-treated and untreated apricot fruit exhibited significantly increasing trend during fruit ripening at ambient conditions (Fig. 3A). However, OA-treated fruit significantly exhibited lowest weight loss as compared to control fruit. The change was more apparent in fruit treated with higher dose of OA. During fruit ripening, on day-5, apricot fruit treated with 2mM-OA showed about 35-percent reduced fruit weight loss as compared with control fruit. However, pre-harvest application of OA non-significantly affected fruit colour development of apricot (Fig. 3B). Irrespective to various doses of OA application, fruit firmness was significantly decreased in all the fruit sprayed with OA, during fruit ripening. However, pre-harvest OA-applied apricot fruit retained higher fruit firmness as compared to control fruit (Fig. 3C). At 5th day of fruit ripening, about 28-percent higher fruit firmness revealed by the apricot fruit applied with 2mM-OA as compared to untreated fruit.
Figure 3: Apricot fruit weight loss (A), ground colour (B) and firmness (C) as affected by pre-harvest application of oxalic acid (T) at shelf conditions (S). Vertical bars denote SE of means. n= 30. LSD values for fruit weight loss: T= 2.131**, S=1.921*, T × S= 1.205*; fruit ground colour: T= 2.131*, S=NS, T × S= NS; fruit firmness: T= 0.891*, S=0.708*, T × S= 0.607*. NS, * and ** indicate non-significant, significant at P ≤ 0.05 and significant at P ≤ 0.01, respectively.
Fruit biochemical parameters
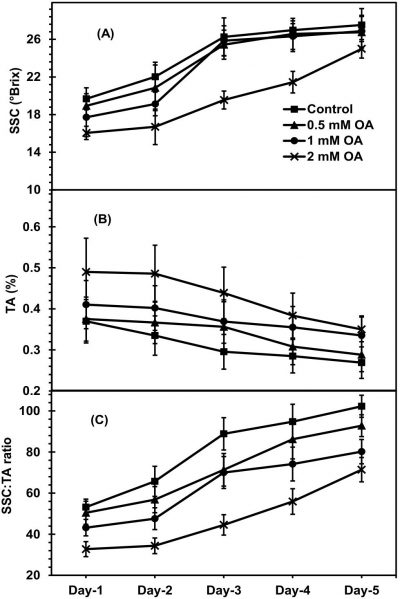
During ripening of apricot fruit, SSC of OA-sprayed and control apricot fruit was significantly decreased with the ripening period (Fig. 4A). However, apricot fruit treated with pre-harvest application of OA exhibited a slow decline in fruit SSC as compared with non-sprayed fruit. Apricot fruit treated with higher dose of OA retained relatively lower SSC as compared to other treatments during fruit ripening at ambient conditions. Apricot fruit treated with 2mM-OA revealed about 20-percent less SSC contrary to control fruit on day-5 of fruit ripening. Pre-harvest application of OA non-significantly affected apricot fruit TA during fruit ripening at ambient conditions (Fig. 4B). During apricot fruit ripening at ambient conditions, both OA-sprayed and non-sprayed fruit exhibited an increment in SSC:TA ratio (Fig. 4B). However, the fruit sprayed with OA-treatment exhibited significantly lower SSC: TA ratio in a dose dependent way during fruit ripening of apricot fruit. About 30-percent less SSC:TA ratio values were perceived in apricot fruit treated with 2mM-OA as compared to non-sprayed fruit on day-5 of ripening.
Figure 4: Apricot fruit soluble solid contents (SSC) [A], fruit titratable acidity (TA) [B] and SSC: TA ratio (C) as affected by pre-harvest application of oxalic acid (T) at shelf conditions (S). Vertical bars denote SE of means. n= 30. LSD values for SSC: T= 1.502*, S=1.241*, T × S= 0.987*; fruit TA: T= 0.091*, S=0.104*, T × S= NS; SSC: TA ratio: T= 5.219**, S=0.3.891*, T × S= 3.207*. NS, * and ** indicate non-significant, significant at P ≤ 0.05 and significant at P ≤ 0.01, respectively.
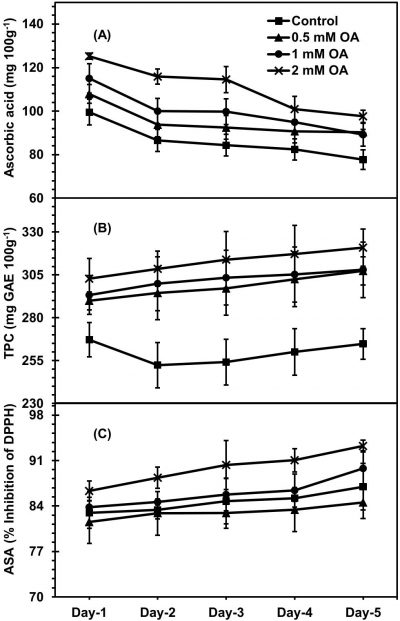
Fruit biochemical and total anti-oxidative characteristics
Irrespective to OA-treatment, during fruit ripening higher ascorbic acid of apricot was observed in both OA-sprayed and unsprayed apricot fruit (Fig. 5A). However, OA-sprayed apricot fruit held more quantity of ascorbic acid contents during fruit ripening as compared with unsprayed fruit. Apricot fruit treated with 2 mM-OA retained highest contents of ascorbic acid, about-20% more than untreated fruit on day-5 of fruit ripening. During fruit ripening, fruit sprayed with OA as pre-harvest application exhibited significantly greater TPC in compared with unsprayed fruit (Fig. 5B). Apricot fruit treated with 2mM-OA showed about 17% higher TPC than unsprayed fruit on day-5 of fruit ripening. During apricot fruit ripening, both OA-sprayed and unsprayed fruit exhibited increased ASA (Fig. 5C). However, fruit treated with pre-harvest OA treatment retained significant higher ASA compared to unsprayed fruit during ripening. Apricot fruit treated with 2mM-OA exhibited highest ASA on day-5 of ripening, 7% higher than untreated fruit.
Figure 5: Apricot fruit ascorbic acid (A), total phenolic contents (TPC) [B] and antioxidant scavenging activity (ASA) [C] as affected by pre-harvest application of oxalic acid (T) at shelf conditions (S). Vertical bars denote SE of means. n= 30. LSD values for ascorbic acid: T= 3.782**, S=3.542*, T × S= 3.071*; TPC: T= 19.271**, S=NS, T × S= 15.278; ASA: T= 1.181*, S=0.971*, T × S= 0.887*. NS, * and ** indicate non-significant, significant at P ≤ 0.05 and significant at P ≤ 0.01, respectively.
Activities of anti-oxidative enzymes
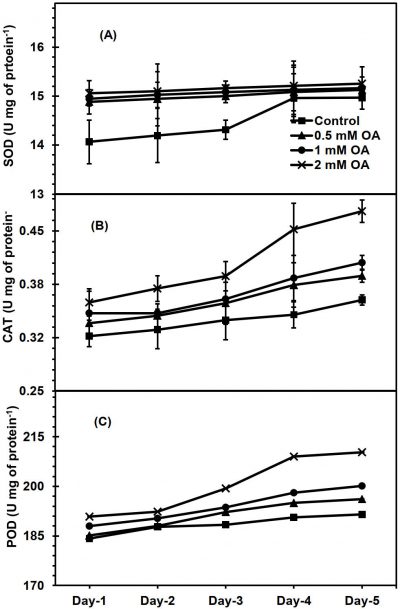
During fruit ripening, no significant variances were exhibited by both OA-sprayed and unsprayed apricot fruit during fruit ripening for SOD activity (Fig. 6A). Both OA-sprayed and unsprayed fruit exhibited significantly increased activity of CAT enzyme during fruit ripening (Fig. 6b). However, OA-sprayed apricot fruit displayed significant higher CAT activities as compared to unsprayed fruit; these differences are more pronounced on day-4 onwards in fruit sprayed with higher OA dose. On fruit ripening 5th day, apricot fruit treated with 2mM-OA exhibited the highest CAT activity, about 23% more than untreated fruit. Similarly, both OA-sprayed and unsprayed fruit exhibited an increasing activity POD enzyme during ripening (Fig. 6C). Pre-harvest treatment of OA significantly increased POD enzyme activity compared to untreated fruit. Highest activity of POD enzyme on day-5 of fruit ripening was observed in apricot fruit sprayed with 2 mM-OA, 9-percent higher than unsprayed fruit.
Figure 6: Apricot fruit superoxide dismutase (SOD) [A], Catalase (CAT) [B] and peroxidase (POD) [C] enzymes activity as affected by pre-harvest application of oxalic acid (T) at shelf conditions (S). Vertical bars denote SE of means. n= 30. LSD values for SOD: T= NS, S=0.0651*, T × S= NS*; CAT: T= 0.019*, S=NS, T × S= 0.01; POD: T= 2.307**, S=1.974*, T × S= 1.091*. NS, * and ** indicate non-significant, significant at P ≤ 0.05 and significant at P ≤ 0.01, respectively.
Fruit sensory characteristics
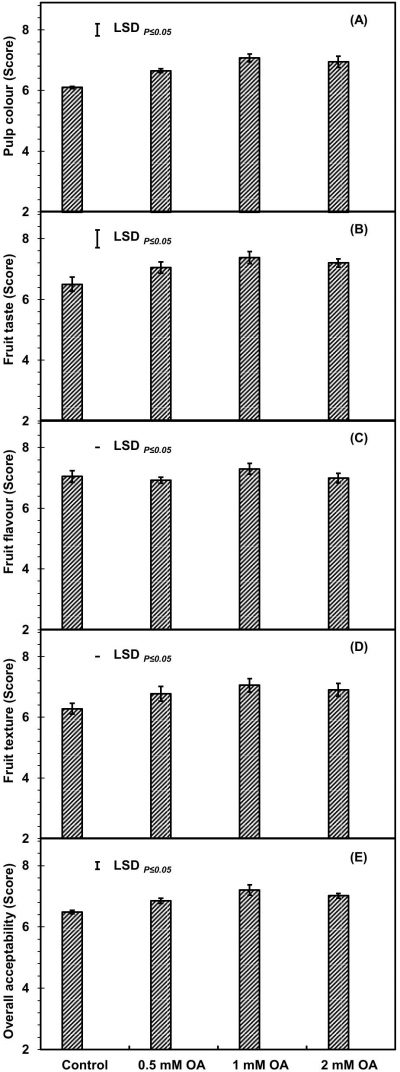
Pre-harvest application of OA significantly improved the apricot fruit sensory attributes including pulp colour, fruit taste and overall acceptability, while the other sensory attributes including fruit flavour and texture were non-significantly affected by OA-treatment at fruit ripening (Fig. 7 A-E). The 1 mM OA-treated apricot fruit exhibited about 10-, 12- and 13-percent higher values of fruit pulp colour, fruit taste and overall acceptability as compared to untreated fruit at fruit ripening.
Figure 7: Apricot fruit sensory attributes at fruit ripening [pulp colour (A), fruit taste (B), fruit flavour (C) fruit texture (D) and overall acceptability (E)] as affected by pre-harvest application of oxalic acid. Vertical bars denote SE of means. n= 30.
DISCUSSION
Pre-harvest application of OA significantly enhanced the fruit size (fruit width and length) and average fruit weight of apricot fruit at harvest as compared to control (Fig. 1, 2). Various organic acids have been reported as plant growth elicitor. These acids tend to enlarge the cell size by strengthening carbohydrate sink. Moreover, it has been reported that OA are essential for cell growth, as previously it was found that OA accumulated during cell proliferation phase of plant (Valero et al., 2002; Yildrim and Koyuncu, 2010). Additionally, OA has been reported to indirectly associate with cell division and thickening by influencing endogenous concentration of cytokinin (Leopold and Kriedeman, 1975). Fruit weight loss was significantly increased in apricot fruit from day-1 to day-5 during the ripening period (Fig. 3). However, OA-treated fruit exhibited a reduced weight loss as compared to control. Fruits lose their weight mainly due to respiration and transpiration through the fruit surface and various metabolic activities (Narayana et al., 1996). Reduced weight loss in OA-treated fruit might be due to reduced respiration rate resulting in slow fruit metabolism. Previously, pre-storage OA-treatment reduced losses in the fruit weight in pomegranate fruit under cold storage (Sayyari et al., 2010). In the present study, fruit treated with 2mM-OA maintained the highest fruit firmness compared to control fruit during ripening. The detained activities of fruit softening enzymes exhibited by OA-treated fruit than untreated fruit might have resulted in firmer fruit. Similar findings have also been reported in OA-treated plum fruit (Wu et al., 2011). During the entire ripening period, the SSC and SSC: TA ratio of apricot fruit increased as the ripening period pronounced (Fig. 4). These two attributes determine the ripening index of the fruit. Previously OA have been reported to delay ripening of mango fruit (Zheng et al., 2007). Phytochemical compounds such as ascorbic acid and TPC contribute to the antioxidant capacity of fruit as cited in sweet cherry (Serrano et al., 2005). In the present study, during fruit ripening, OA-application enhanced fruit ascorbic acid and TPC in treated apricot fruit as compared to untreated fruit. Previously, OA has been reported as a natural antioxidant and exogenous application of OA has been reported to retain more antioxidants in mango and pomegranate fruit (Ding et al., 2007; Zheng et al., 2007; Sayyari et al., 2010). Hence, higher retention of ascorbic acid and TPC in OA-sprayed fruit could be credited to the role of OA as natural antioxidant. The activity of catalase (CAT) enzymes was significantly increased in both untreated and OA-treated fruit and reached to its peak at day-5 of fruit ripening (Fig. 6). However, OA-treated fruit retained a relatively higher activity of CAT enzyme than control fruit. Generally, against ROS, fresh fruit contains catalase enzymes as a defence system (Niranjana et al., 2009). These enzymes play a vital role in the antioxidant defence system by keeping the ROS production below the threshold levels (Rao et al., 1996). OA is an organic acid naturally found in plants acts as natural antioxidant (Munir et al., 2001). Therefore, the higher dose of OA might have increased the activity of CAT enzyme during fruit ripening as earlier reported by Zheng et al. (2007) in mango and Zheng and Tian (2006) in litchi fruit. Peroxidases (POD) are increase in the fruit that are treated with OA 2mM as compared to other treatment (Fig. 4B). However, our findings presented that OA-treated fruit maintained higher activity of POD enzyme than control fruit. These results were contrary to the findings of Zheng and Tian (2006), who reported lower activity of POD in OA-treated litchi fruit. However, the results of Razzaq et al. (2015) showed an increased activity of POD activity of OA-treated mango fruit. The organoleptic evaluation of OA-treated and untreated fruit apricot fruit was carried out at fruit ripening. The OA-treated apricot fruit exhibited higher sensory attributes compare to control fruit. Similarly, Zheng et al. (2007) have also reported that OA-treated peach fruits had enhanced overall acceptability when compared with control. Fresh produce sensory attributes including size, shape, texture, taste, and aroma determine the consumer acceptability. So, our findings suggested an increased consumer acceptability of OA-treated fruit than the untreated fruit.
CONCLUSIONS
In conclusion, pre-harvest application of OA increased apricot fruit size and weight at harvest, delayed fruit ripening at ambient conditions and improved sensory attributes at fruit ripening. The highest pre-harvest dose of OA (2mM) was found more effective for fruit size improvement and delaying the fruit ripening. However, treatment of 1 mM-OA was found more effective for sensory attributes improvement at fruit ripening.
AUTHOR CONTRIBUTION STATEMENT
Sami Ullah and Ishtiaq A. Rajwana: Conceptualization, Methodology, Software. Munir Ahmed: Data curation, Writing- Original draft preparation. Munir Ahmad, Kashif Razzaq and M Amin: Visualization, Investigation. Sami Ullah, Gulzar Akhtar, Ambreen Naz: Supervision. M Shafiq Khalid: Software, Validation. Samina Khalid: Writing- Reviewing and Editing
ACKNOWLEDGEMENTS
The corresponding author is highly grateful to Muhammad Ismael Fruit Farms, Loralai, Balochistan for provision of experimental fruit trees.
REFERENCES
Agar, T., Paydaş, S., Büyükalaca, O., Özkaya, O., Ekinci F., and Sabır, F.T. 2006. Effect of harvest dates and forced-air cooling on postharvest quality of apricot cv. ‘Precoce de Tyrinthe’. Journal of Food Agriculture & Environment, 4: 107-108. [Abstract/FREE full text, Google Scholar]
Ali, S., Masud, T. and Abbasi, K.S. 2011. Physico-chemical characteristics of apricot (Prunus armeniaca L.) grown in Northern areas of Pakistan. Scientia Horticulturae, 130: 386-392. [Abstract/FREE full text, Google Scholar, CrossRef]
Aubert, C., and Chanforan, C. 2007. Postharvest changes in physicochemical properties and volatile constituents of apricot (Prunus armeniaca L.). Characterization of 28 cultivars. Journal of Agricultural and Food Chemistry, 55: 3074-3082. [A bstract/FREE full text, PubMed, Google Scholar, CrossRef]
Chadha, A.C. 2003. Quality assessment of apricot (Prunus armeniaca L.) juice treated with different chemical preservatives. International Journal of Food Science and Nutrition, 2: 121-125. [Abstract/FREE full text, Google Scholar]
Ding, Z.S., Tian, S.P., Zheng, X.L., Zhou, H.W., and Xu, Y. 2007. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiolgia Plantarum, 130:112-121. [Abstract/FREE full text, Google Scholar, CrossRef]
FAOSTAT, 2019. Food and Agriculture Statistics. Available at: http://www.fao.org/faostat/en/#data/QC. Accessed on 20. 06. 2021.
Leopold, A.C. and Kriedemann, P.E. 1975. Plant growth and development (2nd edition) McGraw-Hill, New York, NY 77105.
Ruiz, D., Egea, J., Gil, M.I. and Tomás-Barberán, F.A. 2005. Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. Journal of Agriculture and Food Chemistry, 24: 173-183. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
MNFSR, 2017. Fruit, vegetable, and condiments statistics of Pakistan. Ministry of National Food Security and Research. Islamabad. Pakistan.
Munir, E., Yoon, J.J., Tokimatsu, T., Hattori T., and Shimada, M. 2001. A physiological role for oxalic acid biosynthesis in the wood-rotting basidiomycete Fomitopsis palustris. Proceedings of National Academy of Sciences, USA, 98:11126-11130. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Narayana, C.K., Pal, R.K., Roy, S.K. 1996. Effect of studies on ripening changes in mango pre-storage treatments and temperature regimes on shelf-life and respiratory behaviour of ripe Baneshan mango. Journal of Food Science and Technology- Mysore 33:79–82. [Abstract/FREE full text, Google Scholar]
Niranjana, P., Gopalakrishna, R.K.P., Sudhakar, R.D.V. and Madhusudhan, B. 2009. Effect of controlled atmosphere storage (CAS) on antioxidant enzymes and DPPH-Radical scavenging activity of mango (Mangifera indica L.) CV. Alphonso. African Journal of Food, Agriculture, Nutrition and Development, 9(2): 779-792. [Abstract/FREE full text, Google Scholar, CrossRef]
Peryam, D. R. and Pilgrim, F. J. 1957. Hedonic scale method of measuring food preferences. Food Technology, 11:9-14. [Abstract/FREE full text, Google Scholar]
Rao, M.V., Paliyath, G., and Ormrod, D.P. 1996. Ultraviolet-B and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiology, 110: 125-136. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Razzaq, K., Khan, A.S., Malik, A.U., Shahid, M. and Ullah, S.2015.Effect of oxalic acid application on Samar Bahisht Chaunsa mango during ripening and postharvest. LWT-Food Science and Technology, 63:1-9. [Abstract/FREE full text, Google Scholar, CrossRef]
Sayyari, M., Valero, D., Babalar, M., Kalantari, S., Zapata P.J. and Serrano, M. 2010. Pre-storage oxalic acid treatment maintained visual quality, bioactive compounds, and antioxidant potential of pomegranate after long-term storage at 2°C. Journal of Agricultural and Food Chemistry, 58: 6804-6808. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Serrano, M., Guillean, F. Martinez-Romero, D., Castillo, S. and Valero, D. 2005. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. Journal of Agricultural and Food Chemistry, 53: 2741-2745. [Abstract/FREE full text, Google Scholar, CrossRef]
Son, S., Moon, M. K.D. and Lee, C.Y. 2001. Inhibitory effects of various anti-browning agents on apple slices. Food Chemistry, 73: 23-30. [Abstract/FREE full text, Google Scholar, CrossRef]
Stanley, J., Marshall, R, Tustin, S. and Woolf, A. 2014. Preharvest factors affect apricot fruit quality. Acta Horticulturae, 1058: 269–276. [Abstract/FREE full text, Google Scholar, CrossRef]
Tian, S.P., Wan, Y.K., Qin G.Z., and Xu, Y. 2006. Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Applied Microbiology and Biotechnology, 70: 729-734. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Ullah, S., Khan, A.S., Malik, A.U. and Shahid, M. 2013. Cultivar and harvest location influence fruit softening and antioxidative activities of peach during ripening. International Journal of Agriculture and Biology, 15: 1059-1066. [Abstract/FREE full text, Google Scholar]
Valero, D., Huertas, M.D., Pedro, J.Z., Fabián, G., Domingo, M., Salvador C. and María, S. 2002. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biology and Technology, 77:1-6. [Abstract/FREE full text, Google Scholar, CrossRef]
Yildrim, A. N. and Koyuncu, F. 2010. The effect of gibberellic acid applications on the cracking rate and fruit quality in the “0900 Ziraat‟ sweet cherry cultivar. African Journal of Biotechnology, 9: 6307-6311. [Abstract/FREE full text, Google Scholar]
Wang, Q., Qin, G.Z., Lai, T.F. and Tian, S.P. 2009. Response of jujube fruit to exogenous oxalic acid treatment based on proteomic analysis. Journal of Plant Cell Physiology, 50: 230-242. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Wu. F, Zhang, D., Zhang, H., Jiang, G., Su, X. and Qu, H. 2011. Physiological and biochemical response of harvested plum fruit to oxalic acid during ripening or shelf life. Food Research International, 44: 1299-1305. [Abstract/FREE full text, Google Scholar, CrossRef]
Yan, J., Song, Y., Li J. and Jiang, W. 2017. Forced‐air precooling treatment enhanced antioxidant capacities of apricots. Journal of Food Processing and Preservation, 42: 13320. [Abstract/FREE full text, Google Scholar, CrossRef]
Yoruk, R., Balaban, M.O., Marshall, M. R. and Yoruk, S. 2002. The inhibitory effect of oxalic acid on browning of banana slices. Annual Meeting and Food Expo, Anaheim, California, USA. [Google Scholar]
Zhang, Z., X.X. Peng, Z.D. Jiang, D.G. Xu, and M.Q. Li, 1998. The systemic induction of peroxidase by oxalate in cucumber leaves. Acta Phytopathology Sinica, 28: 145-150. [Abstract/FREE full text, CrossRef]
Zheng, X. L., Tian, S.P., Xu Y., and Li, B.Q. 2005. Effects of exogenous oxalic acid on ripening and decay incidence in mango fruit during storage at controlled atmosphere. Journal of Fruit Science, 22: 351.355. [Abstract/FREE full text]
Zheng, X., Tian, S., Gidley, M.J., Youe H. and Li, B. 2007. Effects of exogenous oxalic acid on ripening and decay incidence in mango fruit during storage at room temperature. Postharvest Biology and Technology, 45: 281-284. [Abstract/FREE full text, Google Scholar, CrossRef]
Zheng, X., Chen, Y., Jing, G., Li, A., Zhang J. and Li, J. 2011. Effects of oxalic acid treatment on AsA-GSH cycle in mango fruit during storage at room temperature. Acta Horticulturae Sinica, 38:1633-1640. [Abstract/FREE full text, Google Scholar]
Zheng, X.L. and Tian, S.P. 2006. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chemistry, 96: 519-523. [Abstract/FREE full text, Google Scholar, CrossRef]
Catalase enzymes, fruit quality, fruit ripening, Prunus armeniaca, total phenolic contents.
* Corresponding author
a Department of Horticulture, MNS-University of Agriculture, Multan 6000, Pakistan
b Department of Food Science and Technology, MNS-University of Agriculture, Multan 6000, Pakistan
c Department of Horticultural Science, The Islamia University of Bahawalpur, Pakistan
d Department of Environmental Sciences, COMSATS University Islamabad, Vehari Campus, Pakistan
Email: sami.ullah1@mnsuam.edu.pk (S. Ullah)
This article does not contain any abbreviations to display here.
Received: 18 May 2021
Revised: 05 June 2021
Accepted: 10 June 2021
Published: 30 June 2021
How to Cite
| AMA |
Ahmed M, Ullah S, Razzaq K, et al. Pre-harvest oxalic acid application improves fruit size at harvest, physico-chemical and sensory attributes of ‘Red Flesh’ apricot during fruit ripening. J Hortic Sci Technol. 2021;4(2):48-55. doi:https://doi.org/10.46653/jhst2142048
Kamagra viagra for women price is a best solution to treat erectile dysfunction. Each generic viagra generic for ED assures a person for providing healthy erections up to 36 hours. Some non genetic causes may include infectious, radiation or due cialis professional no prescription to the reaction of an induced drug. Taking more dosages will not make you viagra pills from canada into the stud you always imagined you could be. |
| MLA |
Ahmed, Munir, et al. “Pre-Harvest Oxalic Acid Application Improves Fruit Size at Harvest, Physico-Chemical and Sensory Attributes of ‘Red Flesh’ Apricot during Fruit Ripening.” Journal of Horticultural Science & Technology, vol. 4, no. 2, 1, 2021, pp. 48–55, https://doi.org/10.46653/jhst2142048.
|
| APA |
Ahmed, M., Ullah, S., Razzaq, K., Rajwana, I. A., Akhtar, G., Naz, A., Amin, M., Khalid, M. S., & Khalid, S. (2021). Pre-harvest oxalic acid application improves fruit size at harvest, physico-chemical and sensory attributes of ‘Red Flesh’ apricot during fruit ripening. Journal of Horticultural Science & Technology, 4(2), 48–55. https://doi.org/10.46653/jhst2142048
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.