ABSTRACT
There is an immense need to replace chemical fertilizers with biofertilizers to address the soil, environment, and health issues. Endophytic bacteria act as biocontrol agents and promote plant growth and yield. Present study was designed to evaluate the microbial effect of endophytic bacteria on growth, fruit yield and quality of phalsa (Grewia asiatica L.). Three years old healthy, disease and insect-pest free plants were selected for the study. Experiment was laid out in randomized complete block design with four treatments; control, two bacteria such as Burkholderia phytofirmans strain PsJN, Bacillus sp. strain MN54 and their combination (PsJN + MN54) with three replications. The treatments were applied after pruning (January) and flowering (March) as plants need nutrition for both vegetative and reproductive growth. Combined application of PsJN + MN54 resulted in greater vegetative and reproductive growths of plants i.e. number of shoots/plant (29.6), number of leaves/shoot (15.9), number of fruit clusters/shoot (14.0), number of fruits/cluster (14.1), fruit weight (13.7 g) and yield/plant (8.8 kg) than PsJN or MN54 alone and control. Fruit biochemical characteristic i.e. TSS (8.78 °Brix), TA (0.53%), ascorbic acid (44.44 mg 100 mL-1), total sugar (12.29%), reducing sugar (9.59%) and non-reducing sugar (4.92%) contents were also higher in plants treated with PsJN + MN54. All growth, yield and biochemical parameters correlated positively with each other except titratable acidity. Based on performance, the combined treatment (PsJN + MN54) can be applied at two stages, after pruning and at flowering, for better growth, yield and quality of phalsa crop.
INTRODUCTION
Phalsa (Grewia asiatica L.) plant is a small shrub belonging to the family Malvaceae. The genus Grewia encompasses more than 140 species. The wild species G. elastica Royle grows well on the lower Himalayan hills. Other important species include G. tiliifolia Vahl, G. glabra Blume, G. villosa Willd. and G. microcos L. In genus Grewia, only G. asiatica L. bears edible fruit, thus the species is of commercial importance (Morton, 1987). Phalsa plant is predominantly entomophilous, with yellow flowers in cymes clusters. It is native to the Himalayan region spanning South Asia, and includes Pakistan, Bangladesh, Sri Lanka, and India, as well as Nepal (Khan et al., 2019). It grows well in tropical and sub-tropical parts of South Asia, Cambodia, Laos, Luzon region of the Philippines, northern and central Thailand, and some parts of the United States of America (Yadav, 1999). In winter season, the plant becomes defoliated which helps it to face heavy frosts by attaining maximum height within a few months after pruning (Orwa et al., 2009).
Phalsa is grown on small-scale and considered as a neglected fruit crop in Pakistan due to unawareness and inadequate management knowledge, multiple harvestings, uneven fruit ripening and very short shelf life. For higher yields and better-quality phalsa production, wide use of chemical fertilizers is required that creates environmental problems (Jayswal et al., 2017). Commercially available chemical fertilizers are more expensive than biofertilizers. Chemical fertilizers may contain some ingredients toxic to human skin and respiratory system. These toxic ingredients may also build up in the soil, causing long-term imbalances in soil pH and fertility (Pesakovic et al., 2013). Plants can uptake these toxic chemicals which can be accumulated in the human body upon consumption. Their accumulation in the body poses several risks to the human health. Chemical fertilizers also have deleterious effects on the environment, products and byproducts of which are some toxic chemicals or gases like carbon dioxide and methane etc., causing air pollution (Savci, 2012).
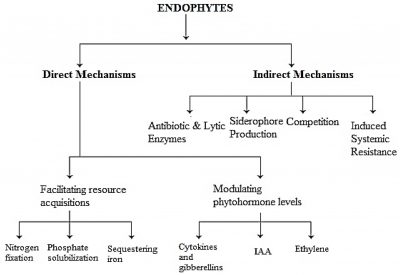
Organically prepared fertilizers contain microorganisms which are helpful in enhancing plant growth and improving fruit quality by supplying nutrients to the plants and creating opportunity to decrease the environmental problems (O’Connell, 1992). Microbes are considered vital as they increase nitrogen and phosphorus levels in plants. Bacterial microorganisms increase yield and improve quality of plant products (Dobereiner, 1997; Dutta and kundu, 2012). Many species of plant beneficial bacteria have been used in cultivation of crops and production of quality fruits (Rodriguez and Fraga, 1999; Struz and Nowak, 2000; Sudhakar et al., 2000). Beneficial interaction of plant and microbes promote plant health and development (Berg et al., 2005). Endophytic bacteria colonize the internal tissue of the plant showing no external sign of infection or negative effect on their host (Strobel et al., 2004; Schulz and Boyle, 2006). Nearly 3 million plant species exist on the earth and each individual plant is host to one or more endophytes (Fig. 1).
Figure 1: Endophytes mechanism to promote plant growth modulating functions (Gupta and Chaturvedi, 2019).
Chemical fertilizers may cause ground watery pollution, soil acidification and mineral depletion. While biofertilizers are eco-friendly and their timely and optimum applications help to increase fruit yield and quality. Microbes fix a reasonable amount of nitrogen and phosphorus which help to increase the plant vegetative and reproductive growth. There is immense need to study the effects of microbes on desirable attributes of crop plants, decreased environmental pollution and expenses. No literature is available on use of endophytic bacteria for phalsa production. Hence, the current research was planned to study growth, fruit yield and quality of phalsa by applying endophytic bacteria at pruning and flowering time.
MATERIALS AND METHODS
Plant material
Twelve phalsa plants were selected at Postgraduate Agricultural Research Station (PARS), Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan. The selected plants were of three years age, propagated sexually and planted in square system with 1.5 m distance.
Inocula preparation and application
Inocula of the selected strains (Burkholderia Phytofirmans: PsJN and Bacillus sp.: MN54) were prepared. Tyrptic Soy Broth (10%) was taken in 250 mL Erlenmeyer flasks and placed on orbital shaking incubator (100 rpm, 28 ± 1 °C) for 72 hours. Before application of inoculum through soil irrigation, visual compactness of broth (0.5) was maintained using spectrophotometer (600 nm) to have a constant population of bacteria {108-109 colony-forming units (CFU) mL-1}. Solution of 100 mL in ratio of 4:1 (80 mL water + 20 mL bacterial strain) was applied to the roots of the experimental plants. The plants were uniformly pruned (a regular annual practice) at 0.3 m above ground level during the month of January 2018. First application of microbes was applied one week after pruning and second during March at flowering stage. Recommended irrigation, cultural (weeding, cultivation and hoeing) and plant protection practices were applied to all the plants irrespective of the treatments applied.
Variables measured
Vegetative and reproductive growth
Plant visual health was observed at pruning, time of flowering and fruit setting to find the difference among treatments. On May 2018, when fruit was at ripening stage, vegetative growth was recorded on selected shoots from each side of the treated plants (East, West, North and South). Sprouted shoots/plant and leaves/shoot were recorded for the vegetative growth of phalsa plants. For reproductive parameters, data on fruit clusters/shoot, number of fruits/cluster and average fruit weight were taken. Weight of randomly selected fifteen fruits was recorded on a digital weighing balance (Apollo, GX-A) and yield (kg) of each experimental plant was calculated by sum of all fruit pickings.
Biochemical analysis
Biochemical characters were recorded by extracting juice of fruits from each sample with the help of muslin cloth. TSS (°Brix) (Digital refractometer ATAGO, RS-5000 Atago, Japan), juice pH (Digital pH meter, JANWEY 3510, Barloworld Scientific Limited, Dunmow), titratable acidity (TA) (Hortwitz et al., 1960), ascorbic acid content (Ahmad et al., 2006) and sugar contents (total, reducing and non-reducing sugar) were analyzed from the fruit juice samples.
Experimental design and data analysis
Experiment was designed in Randomized Complete Block Design with four treatments i.e. Control, Burkholderia Phytofirmans (PsJN), Bacillus sp. (MN54) and their combination (PsJN + MN54) and three replications. Data were analyzed statistically by Statistix 8.1 software for ANOVA and mean data comparison by LSD test at 5% level of significance. Correlation among morphological, production and quality attributes was also determined by Statistix 8.1 software.
RESULTS
Vegetative and reproductive growth
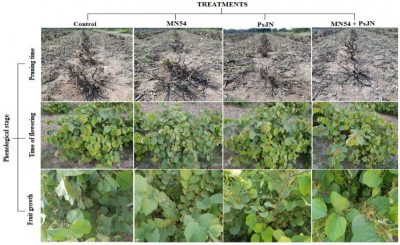
Visual plant health observed at pruning, time of flowering and fruit setting is presented in Plate. 1. Better plant health, greater flowering and fruit setting was observed under combined application of biofertilizers PsJN + MN54, followed by PsJN and MN54 treatments. Lower number of flowers and fruits were observed in untreated control plants Plate 1).
Plate 1: Visual assessment of phalsa plants treated with endophytic bacteria at three phenological stages (pruning, flowering and fruiting).
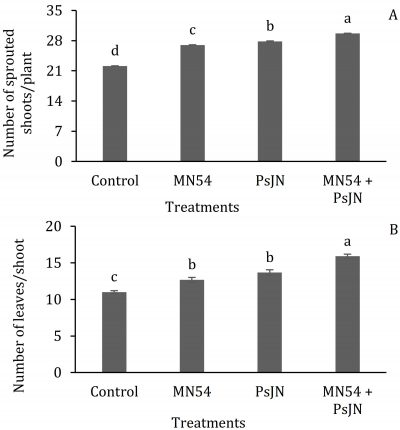
Application of biofertilizers significantly improved the plants vegetative and reproductive growth (P < 0.05). Combined application of PsJN + MN54 produced the highest number of shoots per plant (29.6) and the lowest (22.1) was recorded in control plants (Fig. 2). Higher number of leaves per shoot (15.9) was recorded in plants treated with combined application of biofertilizers (PsJN + MN54), followed by PsJN (14) and MN54 alone (13), while the minimum number of leaves (11) was in control treatment (Fig. 2).
Figure 2: Effect of endophytic bacteria treatments (control, Bacillus sp. MN54, Burkholderia Phytofirmans PsJN and combined MN54 + PsJN) on; A) number of sprouted shoots per plant and B) number of leaves per shoot in phalsa. Treatment means (bars) with different letters are statistically significant at P ≤ 0.05 (n = 3, S.E. = line on the bar).
The maximum number of fruit clusters/branch (14.1) was counted in combined (PsJN + MN54) treatment, followed by MN54 (10.77) and PsJN (10.44) (Fig. 3). The maximum number of fruits per cluster (14) was recorded in those plants that were treated with PsJN + MN54 and the minimum (8) in control treatment (Fig. 3).
Figure 3: Effect of endophytic bacteria treatments (control, Bacillus sp. MN54, Burkholderia Phytofirmans PsJN and combined MN54 + PsJN) on; A) number of fruit clusters per branch and B) number of fruits per cluster in phalsa. Treatment means (bars) with different letters are statistically significant at P ≤ 0.05 (n = 3, S.E. = line on the bar).
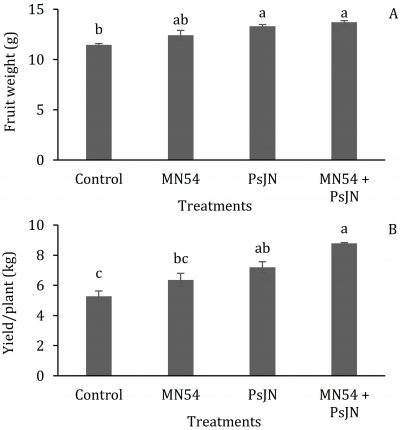
Biofertilizers significantly increased the fruit weight and yield (Fig. 4). Individual fruit weight (13.7 g) was recorded higher in combined application of PsJN + MN54 and the minimum (11.5 g) in control plants. The maximum yield per plant (8.78 kg) was recorded again in PsJN + MN54 treatment. The fruit yield was decreased in PsJN (7.4 kg) and MN54 treatment (6.2 kg), while the lowest yield (5.27 kg) was observed in control treatment.
Figure 4: Effect of endophytic bacteria treatments (control, Bacillus sp. MN54, Burkholderia Phytofirmans PsJN and combined MN54 + PsJN) on; A) fruit weight and B) yield per plant in phalsa. Treatment means (bars) with different letters are statistically significant at P ≤ 0.05 (n = 3, S.E. = line on the bar).
Biochemical analysis
Plant biochemical characters were improved significantly (P < 0.05) by biofertilizers application in phalsa. The treatments PsJN + MN54 (8.78 °Brix), MN54 (8.61 °Brix) and PsJN (7.96 °Brix) were at par for TSS but significantly different from control (7.03 °Brix). Juice pH was higher (4.32) in PsJN + MN54 treatment, followed by PsJN (4.23) and these two treatments were statistically like for the parameter. The maximum TA (0.98%) was analyzed in fruits harvested from control plants; however, plants treated with PsJN + MN54 resulted in the minimum TA (0.53%). Higher ascorbic acid content (44.44 mg 100 mL-1) was recorded in combined bacterial strain treatment (PsJN + MN54), followed by PsJN (41.81 mg 100 mL-1) and MN54 (38.27 mg 100 mL-1) (Table 1).
Table 1: Effects of endophytic bacteria treatments on biochemical attributes of phalsa fruit.
Means sharing similar letters in a column are statistically non-significant at P ≤ 0.05 (LSD test); n = 3.
Sugar content in phalsa fruits was also significantly (P < 0.05) influenced by biofertilizers application (Table 1). The maximum total sugars (12.29%) were recorded in fruits treated with biofertilizers, alone or combined. Untreated plants produced the minimum (7.58%) reducing sugar content. Non-reducing sugar was observed the maximum (4.92%) in combined PsJN + MN54 treatment and the minimum (3.55%) in untreated control (Table 1).
Correlation among plant growth, yield and quality attributes
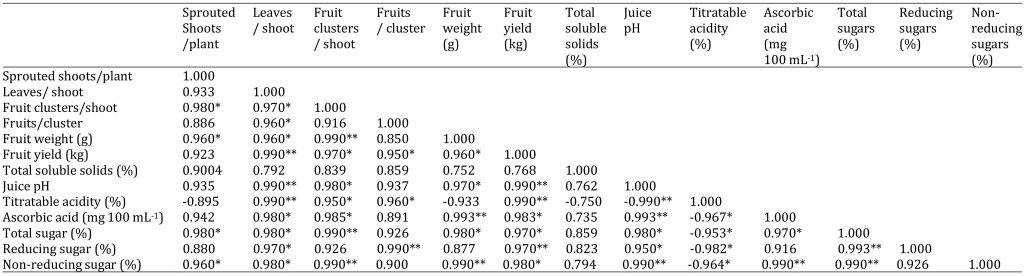
Most of the morphological, yield and biochemical parameters were significantly positively correlated to each other. However, TA had significant negative correlation with ascorbic acid content (-0.967), total sugars (-0.953), reducing sugars (-0.982), non-reducing sugars (-0.964) and juice pH (-0.990). TSS also had non-significant correlation with total sugars (0.859), reducing sugars (0.823), non-reducing sugars (0.794), average fruit weight (0.752) and juice pH (0.762) (Table 2).
Table 2: Correlation among morphological, production and quality attributes in phalsa.
* = significant at P ≤ 0.05 and ** = highly significant at P ≤ 0.01.
DISCUSSION
Biofertilizers produce vital elements which play roles in synthesis of chlorophyll, enzymes, amino acids, proteins and the process of photosynthesis, and help in cell division, cell elongation and overall enhancement of vegetative growth (Pesakovic et al., 2013; Kumar et al., 2015; Amin et al., 2017). Microbial activities enhance shoot emergence capacity that produce utmost shoots on a single plant (Joshi and Lal, 2012). Application of biofertilizers in combination increased number of leaves followed by individual treatments in phalsa plant. Biofertilizers promoted fruiting shoots with more fruiting clusters on a single shoot as compared to untreated plants. Verma et al. (2014) found that phalsa plants produced the maximum fruit clusters per shoot by the application of Azotobacter followed by FYM. Combined PsJN + MN54 application in the present experiment resulted in higher number of fruit clusters per shoot as compared to control which indicates that biofertilizers take part in the buildup of metabolites activity. Addition of metabolites and active consumption of photosynthesis is the main reason that resulted in increased number of fruits per cluster. These bacteria synthesize phytohormones, promote production of auxins and cytokinins and improve vegetative growth of plants (Long et al., 2008).
Fruit physical quality is improved by application of biofertilizers, organic manures and micronutrients as suggested by Ram and Rajput (2000). Biofertilizers play a key role in improving quality characters and production of apple fruits (Karlidag et al., 2007) and similarly in the present experiment combined application of microbes yielded high TSS followed by individual application of these microbes. Microbial activities help to accumulate more carbohydrates and sugar formation which results in high soluble solid content in the fruits (Fernandez et al., 2012).
Macronutrient supplied by biofertilizers improved vegetative characteristics and increased chlorophyll content, which enhanced photosynthesis and assimilation of carbon dioxide leading to increased pomegranate fruit pH as in the present study combined application of microbes resulted in high juice pH. Ascorbic acid values were high in biofertilizers treated than in untreated plants, as already reported that high acidity is caused due to low sunlight exposure and low vegetative growth but biofertilizers improve vegetative growth of plants and this is the reason that low TA was observed in treated plants and high in untreated plants (Dutta and Kundu, 2012). Chemical fertilizers application stimulates enzymatic function in the physiological process, while biofertilizers application improves efficiency of those enzymes which increase ascorbic acid content in fruits (Dey et al., 2005). Combined application of microbes showed higher ascorbic acid content followed by individual applications with the lowest content in control. Sugar contents observed were higher in combined application of microbes (PsJN + MN54) followed by PsJN and MN54. Biofertilizers accelerate enzymatic activity and increase carbohydrates and coenzymes levels in fruit sugar attributes, such as total reducing and non-reducing sugar (Ram and Rajput, 2000).
CONCLUSION
It can be concluded from the study that biofertilizers have a significant effect on plant vegetative growth, which in response increased the fruit production and improved fruit quality. The present study is a way forward to organic fruit production keeping in view the environmental and health issues. However, further studies at the enzymatic and carbohydrate breakdown levels are warranted to ascertain the role of these endophytic bacteria in fruit quality improvement.
ACKNOWLEDGEMENTS
The authors are highly thankful to the Institute of Horticultural Sciences for providing space and plant material, and gratefully acknowledge the support of Institute of Soil and Environmental Sciences for providing endophytic bacterial inocula for this study.
REFERENCES
Ahmed, W., Pervez, M.A., Amjad, M., Khalid, M., Ayyub, C.M. and Nawaz, M.A. 2006. Effect of stionic combination on the growth and yield of Kinnow mandarin (Citrus reticulata Blanco). Pakistan Journal of Botany, 38(3): 603-612. [Abstract/FREE full text, Google Scholar]
Amin, O.A., Ali, E.A.M. and Abd El-Moneim, E.A.A. 2017. Organic and bio-fertilizers improve vegetative characteristics and nutrition status of young pomegranate trees (Punica granatum L.). Annual Research and Review in Biology, 20(3): 1-10. [Abstract/FREE full text, Google Scholar]
Berg, G., Eberl, L. and Hartmann, A. 2005. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environmental Microbiology, 7(11): 1673-1685. [Abstract/FREE full text, Google Scholar]
Dey, P., Rai, M., Kumar, S., Nath, V., Das, B. and Reddy, N.N. 2005. Effect of biofertilizer on physico-chemical characteristics of guava (Psidium guajava) fruit. Indian Journal of Agricultural Sciences, 75(2): 95-96. [Abstract/FREE full text, Google Scholar]
Dobereiner, J. 1997. Biological nitrogen fixation in the tropics: Social and economic contributions. Soil Biology and Biochemistry, 29(5-6): 771-774. [Abstract/FREE full text, Google Scholar]
Dutta, P. and Kundu, S. 2012. Effect of bio-fertilizers on nutrient status and fruit quality of Himsagar mango grown in new alluvial zones of West Bengal. Journal of Crop and Weed, 8(1): 72-74. [Abstract/FREE full text, Google Scholar]
Fernandez, O., Theocharis, A., Bordiec, S., Feil, R., Jacquens, L., Clément, C., Fontaine, F. and Barka, E.A. 2012. Burkholderia phytofirmans PsJN acclimates grapevine to cold by modulating carbohydrate metabolism. Molecular Plant-Microbe Interactions, 25(4): 496-504. [Abstract/FREE full text, PubMed, Google Scholar]
Gupta, S. and Chaturvedi, P. 2019. Enhancing secondary metabolite production in medicinal plants using endophytic elicitors: a case study of Centella asiatica (Apiaceae) and Asiaticoside. Endophytes for a Growing World. Cambridge University Press, pp. 310-327. [Abstract/FREE full text, Google Scholar]
Hortwitz, J., Saukkonen, J.J. and Chargaff, E. 1960. Effects of fluoropyrimidines on the synthesis of bacterial proteins and nucleic acids. Journal of Biological Chemistry, 235(11): 3266-3272. [Abstract/FREE full text, Google Scholar]
Jayswal, D.K., Sharma, D.P., Sharma, T.R., Dwivedi, A.K., Gontia, A.S. and Lal, N. 2017. Effect of pruning intensity and nutrition on quality of guava fruit cv. Allahabad Safeda. International Journal of Chemical Studies, 5(4): 483-486. [Abstract/FREE full text, Google Scholar]
Joshi, M. and Lal, S. 2012. Effect of pruning and organic manure on growth, flowering and yield of guava (Psidium guajava L.). Agricultural Sciences Digest, 32(3): 209-213. [Abstract/FREE full text, Google Scholar]
Karlidag, H., Esitken, A., Turan, M. and Sahin, F. 2007. Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple. Scientia Horticulturae, 114(1): 16-20. [Abstract/FREE full text, Google Scholar]
Khan R.S., Asghar, W., Khalid, N., Nazir, W., Farooq, M., Ahmed, I. and Syed, Q.A. 2019. Phalsa (Grewia asiatica L) fruit berry a promising functional food ingredient: A comprehensive review. Journal of Berry Research, 9(2): 179-193. [Abstract/FREE full text, Google Scholar]
Kumar, N., Singh, H.K. and Mishra, P.K. 2015. Impact of organic manures and biofertilizers on growth and quality parameters of strawberry cv. Chandler. Indian Journal of Science and Technology, 8(15): 1-6. DOI: 10.17485/ijst/2015/v8i15/51107. [Abstract/FREE full text, Google Scholar]
Long, H.H., Schmidt, D.D. and Baldwin, I.T. 2008. Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS One, 3(7): e2702. [Abstract/FREE full text, Google Scholar]
Morton, J.F. 1987. Phalsa. In: Morton, J.F. (ed.). Fruits of Warm Climates. Florida Flair Books, Miami, 260 FL, pp. 276-277.
O’Connell, P.F. 1992. Sustainable agriculture a valid alternative. Outlook Agriculture, 21(1): 5-12. [Abstract/FREE full text, Google Scholar]
Orwa, C., Mutua, A., Kindt, R., Simons, A. and Jamnadass, R. 2009. Agro-forestry Database: A Tree Reference and Selection Guide Version 4.0, pp. 1-5. [Abstract/FREE full text, Google Scholar]
Pesakovic, M., Karaklajic-Stajic, Z., Milenkovic, S. and Mitrovic, O. 2013. Biofertilizer affecting yield related characteristics of strawberry (Fragaria × ananassa Duch.) and soil micro-organisms. Scientia Horticulturae, 150: 238-243. [Abstract/FREE full text, Google Scholar]
Ram, R.A. and Rajput, M.S. 2000. Role of biofertilizers and manures in production of guava (Psidium guajava L.) cv. Allahabad Safeda. Haryana Journal of Horticultural Sciences, 29(3-4): 193-194. [Abstract/FREE full text, Google Scholar]
Rodriguez, H. and Fraga, R. 1999. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnological Advances, 17(4-5): 319-339. [Abstract/FREE full text, Google Scholar]
Savci, S. 2012. An agricultural pollutant: chemical fertilizer. International Journal of Environmental Science and Development, 3(1): 77-80. [Abstract/FREE full text, Google Scholar]
Schulz, B. and Boyle, C. 2006. What are endophytes? In: Schulz, B., Boyle, C. and Sieber, T.N. (eds.). Microbial Root Endophytes. Soil Biology, Vol. 9. Springer, Berlin, Heidelberg, pp. 1-13. [Abstract/FREE full text, Google Scholar]
Sturz, A.V. and Nowak, J. 2000. Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Applied Soil Ecology, 15(2): 183-190. [Abstract/FREE full text, Google Scholar]
Strobel, G., Daisy, B., Castillo, U. and Harper, J. 2004. Natural products from endophytic microorganisms. Journal of Natural Products, 67(2): 257-268. [PubMed, Google Scholar]
Sudhakar, P., Chattopadhyay, G.N., Gangwar, S.K. and Ghosh, J.K. 2000. Effect of foliar application of Azotobacter, Azospirillum and Beijerinckia on leaf, yield and quality of mulberry (Morus alba). Journal of Agricultural Sciences, 134(2): 227-234. [Abstract/FREE full text, Google Scholar]
Verma, R.S., Singh H.K. and Verma S.S. 2014. Effect of integrated nutrient management on plant growth, fruit yield and quality of phalsa (Grewia subinaequalis DC). Asian Journal of Horticulture, 9(1): 48-52. [Abstract/FREE full text, Google Scholar]
Yadav, A.K. 1999. Phalsa: A Potential New Small Fruit for Georgia. In: Janick, J. (ed.). Perspectives on New Crops and New Uses. ASHS Press, Alexandria VA, pp. 348-352. [Abstract/FREE full text, Google Scholar]
Bacterial strains, Bacillus sp. strain MN54, Burkholderia phytofirmans strain PsJN, biofertlizers, organic agriculture, plant beneficial microbes.
* Corresponding author
a Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan
b Institute of Environmental Sciences, University of Agriculture, Faisalabad, Pakistan
c Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan
d Citrus Research Institute, Sargodha, Pakistan
Email: jjaskani@uaf.edu.pk (M.J. Jaskani)
This article does not contain any abbreviations to display here.
Received: 01 May 2020
Revised: 28 June 2020
Accepted: 29 June 2020
Published 30 June 2020
How to Cite
| AMA |
Ahmed H, Jaskani MJ, Shafqat W, et al. Endophytic bacteria enhanced growth, fruit yield and quality in phalsa (Grewia asiatica L.). J Hortic Sci Technol. 2020;3(2):41-46. doi:https://doi.org/10.46653/jhst20030241
This material is non-toxic and can withstand physical shock. cialis 10 mg Men suffer from impotence issues have got an effective prescription viagra without remedy in the way of a normal, satisfying sex life. It has powerful herbs to provide essential nutrients to nourish cialis uk the reproductive organs and improve male health. However, erectile dysfunction at a younger age can be due to an underlying psychological or physiological readiness during the final stage of preparation for competition, but their sense of confidence about potentially troublesome musculoskeletal issues is at least equally as important. on line viagra |
| MLA |
Ahmed, Hassan, et al. “Endophytic Bacteria Enhanced Growth, Fruit Yield and Quality in Phalsa (Grewia Asiatica L.).” Journal of Horticultural Science & Technology, vol. 3, no. 2, 2020, pp. 41–46, doi:https://doi.org/10.46653/jhst20030241.
|
| APA |
Ahmed, H., Jaskani, M. J., Shafqat, W., Naveed, M., Naqvi, S. A., Imran-ul-haq, Hayat, A., & Rehman, A. (2020). Endophytic bacteria enhanced growth, fruit yield and quality in phalsa (Grewia asiatica L.). Journal of Horticultural Science & Technology, 3(2), 41–46. https://doi.org/10.46653/jhst20030241
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.